Table of contents
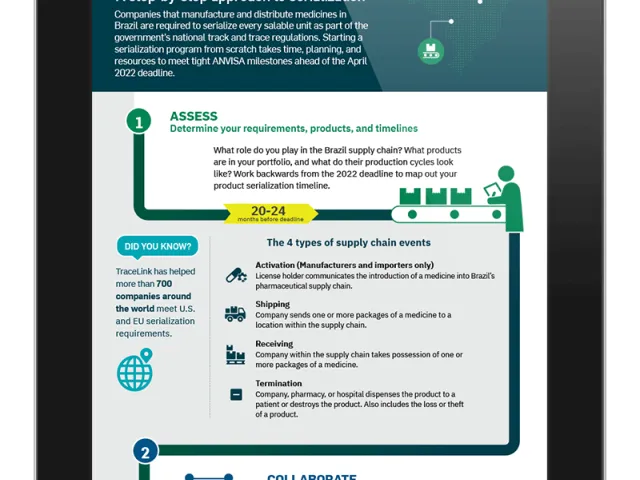
If your company is part of the pharmaceutical supply chain in Europe, preparing for the EU Falsified Medicines Directive should now be a priority. The regulation presents considerable challenges and responsibilities—serialization, government reporting, verification—so understanding the requirements and knowing how to prepare for the February 2019 deadline is a must.
Our latest infographic can get you started on the road to EU FMD compliance. It covers the regulations' essential facts, including which nations are affected—and which have a compliance grace period—what each supply chain company must do to comply, and other vital details about:
- Which products can be grandfathered.
- The 2D DataMatrix code format.
- The specific data that must be uploaded and maintained.